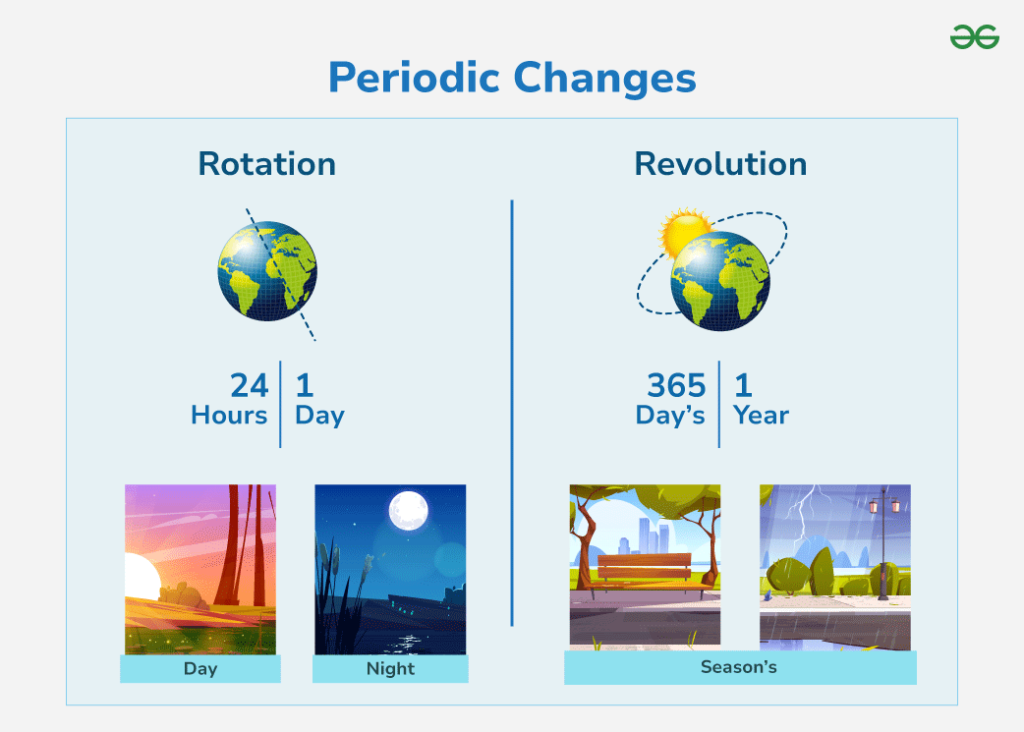
Have you ever wondered how everyday activities can transform materials without altering their chemical structure? Understanding the concept of physical change is key to grasping basic science principles. Identifying examples of physical changes in your surroundings not only sparks curiosity but also deepens your appreciation for the world around you.
Understanding Physical Changes
Physical changes involve alterations to materials without changing their chemical composition. Recognizing these changes in everyday life enhances your understanding of basic science concepts.
Definition of Physical Change
A physical change refers to a process where the form or appearance of a substance changes, but its identity remains the same. For example, when you freeze water into ice, it’s still H2O; just in a different state. Common examples include melting ice, dissolving sugar in water, and chopping wood. These actions do not alter the underlying substances.
Characteristics of Physical Changes
Physical changes exhibit specific characteristics that help identify them:
- Reversibility: Many physical changes can be reversed, like freezing and melting water.
- No New Substances Formed: The original material maintains its chemical properties.
- Energy Changes: While energy may be involved (e.g., heat during melting), it doesn’t create new substances.
- Shape and Size Alterations: You can change physical attributes without affecting composition; for instance, breaking glass or cutting paper.
Recognizing these characteristics aids in distinguishing physical changes from chemical ones in various contexts.
Examples of Physical Changes
Physical changes occur frequently in everyday life. Understanding these changes helps identify them around you. Here are some common examples.
Common Physical Changes
- Freezing water transforms liquid into solid ice while maintaining its chemical structure.
- Melting ice turns solid back into liquid without any change in composition.
- Dissolving sugar in water results in a mixture, but the sugar retains its identity.
- Chopping wood alters its shape and size without changing the wood’s chemical properties.
Everyday Situations
You encounter physical changes daily. For instance, when you cook pasta, it absorbs water and expands, yet remains pasta. Similarly, tearing paper alters its form but not its material makeup. Additionally, mixing sand and salt creates a blend with no new substances formed. Think about how ice cubes melt in your drink; they change state but aren’t transformed chemically. Recognizing these situations enhances your understanding of physical changes around you.
Examples of Non-Physical Changes
Non-physical changes involve transformations that alter the chemical structure of substances. Understanding these kinds of changes is crucial for identifying how materials interact and react in different environments.
Chemical Changes Explained
Chemical changes occur when substances undergo a transformation that results in new substances being formed. For example:
- Rusting iron: Exposure to oxygen and moisture leads to rust, changing the iron’s properties.
- Baking bread: Yeast ferments sugars, producing carbon dioxide and creating a new texture and flavor.
- Burning wood: Combustion produces ash, smoke, and gases, fundamentally altering the original material.
These examples demonstrate how chemical changes produce distinct outcomes that differ from the original substances.
Distinguishing Between Physical and Chemical Changes
You can easily distinguish physical changes from chemical ones by observing key characteristics. Here are some differences:
- Substance identity: Physical changes leave the substance’s identity intact. In contrast, chemical changes create entirely new substances.
- Reversibility: Many physical changes are reversible; for instance, freezing water back into ice. However, reversing chemical changes often isn’t possible without additional processes.
- Energy involvement: Physical changes may require or release energy but do not alter substance composition. On the other hand, chemical reactions generally involve significant energy shifts as bonds break and form.
By recognizing these distinctions, you can better understand everyday phenomena around you.
Importance of Recognizing Physical Changes
Recognizing physical changes plays a vital role in understanding everyday processes and materials. It helps you identify how substances interact in your environment without altering their chemical makeup.
Applications in Real Life
Physical changes occur frequently in daily activities. For instance:
- Cooking: Boiling pasta transforms its shape and texture, yet the composition remains the same.
- Freezing Water: When water freezes into ice, it retains its identity as H2O.
- Tearing Paper: The paper’s form changes, but it’s still paper despite the tearing.
These examples illustrate that even simple actions can involve significant physical alterations while maintaining substance identity.
Impact on Science Education
Understanding physical changes enhances science education by providing foundational knowledge. Students learn to distinguish between physical and chemical changes through practical examples. This distinction is crucial because:
- Encourages Curiosity: Students ask questions about the world around them.
- Promotes Critical Thinking: Identifying examples improves analytical skills.
- Builds a Strong Base for Advanced Concepts: Grasping basic principles lays groundwork for more complex scientific topics.
By focusing on these aspects, learners gain a deeper appreciation for science and its relevance to everyday life.