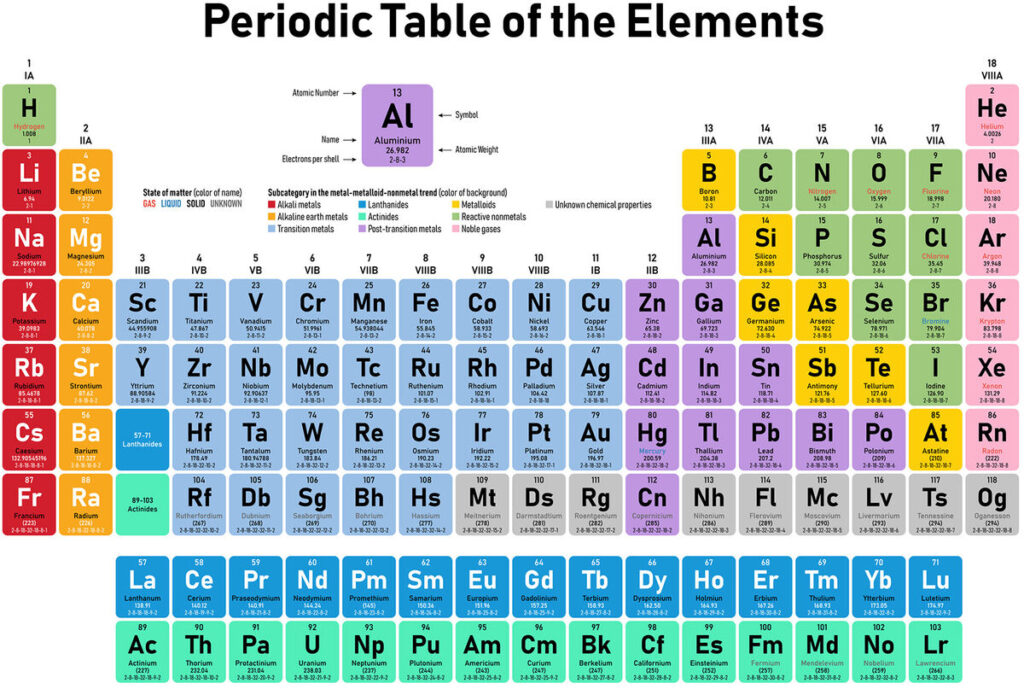
Have you ever wondered what makes up everything around you? The answer lies in the elements on the periodic table. These fundamental building blocks of matter are essential for understanding chemistry and the world we live in. Each element has unique properties that determine how it interacts with others, forming everything from water to complex organic molecules.
Overview of the Periodic Table
The periodic table organizes elements based on their atomic number, electron configuration, and recurring chemical properties. Each element is represented by a unique symbol, which often reflects its name in English or Latin.
The periodic table consists of 118 confirmed elements. These range from hydrogen (H), with an atomic number of 1, to oganesson (Og), which has an atomic number of 118. Elements are grouped into vertical columns called groups and horizontal rows known as periods.
In groups, you’ll find elements that share similar characteristics. For example:
- Group 1: Alkali metals like lithium (Li) and sodium (Na).
- Group 17: Halogens such as fluorine (F) and chlorine (Cl).
Periods indicate the increasing atomic numbers across the table. For instance, moving from left to right in period 2 reveals lithium (Li), beryllium (Be), boron (B), carbon (C), nitrogen (N), oxygen (O), fluorine (F), and neon (Ne).
Transition metals, located in the center of the table, include iron (Fe) and copper (Cu). These metals exhibit valuable properties like conductivity and malleability.
Metalloids, found along the zig-zag line between metals and nonmetals, demonstrate mixed characteristics. Silicon (Si) serves as a prime example due to its semi-conductive nature essential for electronics.
Understanding these classifications helps grasp how different elements interact in chemical reactions to form compounds vital for life.
Main Categories of Elements
The periodic table categorizes elements into three main groups: metals, nonmetals, and metalloids. Each category has distinct characteristics that influence their applications and interactions.
Metals
Metals are typically shiny, malleable, and good conductors of heat and electricity. You can find them on the left side and in the center of the periodic table. Examples include:
- Iron (Fe): Essential for steel production.
- Copper (Cu): Commonly used in electrical wiring.
- Gold (Au): Valued for its rarity and conductivity.
These metals exhibit high density and often form alloys with other elements to enhance properties.
Nonmetals
Nonmetals contrast sharply with metals. They tend to be dull, brittle when solid, and poor conductors. Positioned on the right side of the periodic table, they include:
- Oxygen (O): Crucial for respiration in living organisms.
- Carbon (C): A fundamental building block of life found in all organic compounds.
- Nitrogen (N): Makes up about 78% of Earth’s atmosphere.
Their diverse properties lead to various applications across biological systems and industrial processes.
Metalloids
Metalloids possess characteristics intermediate between metals and nonmetals. They display mixed properties like conductivity that makes them useful as semiconductors. Key examples are:
- Silicon (Si): Widely used in computer chips.
- Boron (B): Important in glassmaking and detergents.
- Arsenic (As): Utilized in some alloys but toxic at higher levels.
Understanding these categories helps you grasp how different elements interact chemically, leading to countless substances essential for daily life.
Understanding Element Groups
Element groups in the periodic table categorize elements based on shared properties, influencing their behavior and interactions. Each group has specific characteristics that define its members.
Alkali Metals
Alkali metals include lithium (Li), sodium (Na), and potassium (K). These elements are highly reactive and typically found in nature combined with other elements. They have low melting points and are soft enough to be cut with a knife. Their reactivity increases as you move down the group, making cesium (Cs) one of the most reactive.
Alkaline Earth Metals
Alkaline earth metals consist of beryllium (Be), magnesium (Mg), and calcium (Ca). These metals are less reactive than alkali metals but still react with water, though not as vigorously. They possess higher melting points than alkali metals, and compounds like calcium carbonate occur naturally in chalk and limestone.
Transition Metals
Transition metals include iron (Fe), copper (Cu), and gold (Au). Known for their ability to form various oxidation states, these metals exhibit unique properties such as high conductivity and malleability. They’re often used in construction materials, electrical wiring, and jewelry due to their strength and durability.
Halogens
Halogens comprise fluorine (F), chlorine (Cl), bromine (Br), and iodine (I). These elements are highly electronegative, meaning they readily gain electrons during chemical reactions. Halogens exist as diatomic molecules in nature; for example, chlorine is found as Cl2 gas. Their reactivity decreases down the group, making astatine (At) less reactive than fluorine.
Noble Gases
Noble gases include helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn). These gases are known for their lack of reactivity due to having complete outer electron shells. As a result, noble gases find applications in lighting—neon lights—and inert environments for sensitive chemical reactions without interference from other gases.
Importance of Elements in Daily Life
Elements play a critical role in your everyday experiences, impacting everything from health to technology. Understanding these elements helps you recognize their significance.
For instance, oxygen (O) is essential for respiration and supports life. Without it, survival becomes impossible. You also rely on carbon (C) as it forms the backbone of organic molecules in food and fuels.
In the realm of technology, metals like copper (Cu) are vital for electrical wiring due to their excellent conductivity. Moreover, silicon (Si) is crucial in semiconductors found in computers and smartphones.
Additionally, elements contribute to household products. For example:
- Sodium (Na) is a key ingredient in table salt.
- Calcium (Ca) strengthens bones and teeth.
- Chlorine (Cl) disinfects drinking water.
You might not notice these elements daily, but they form the basis of countless interactions that enhance your quality of life.