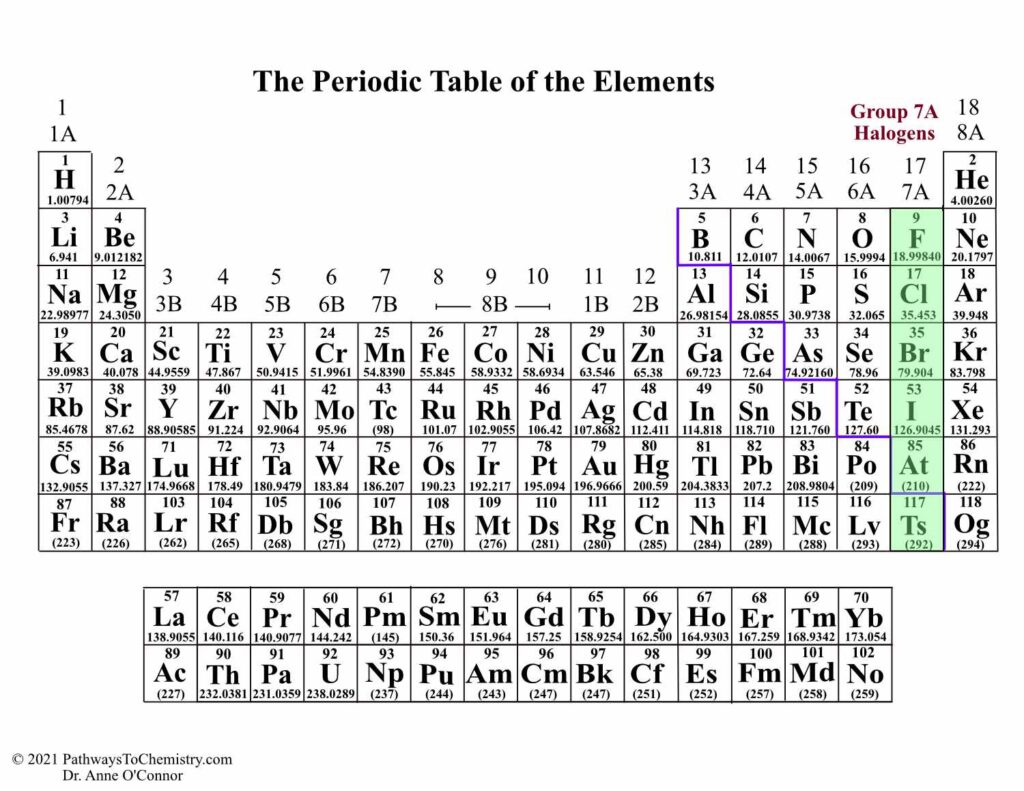
The periodic table is a treasure trove of information, but have you ever wondered what 1A, 3B, and 7A examples represent on it? Understanding these group numbers can unlock the secrets of chemical behavior and element properties. Each designation reveals important insights into how elements interact with one another.
Overview of the Periodic Table
The periodic table organizes elements based on their properties and atomic structure. Group numbers indicate vertical columns, and each group shares similar chemical behaviors. For instance, 1A includes alkali metals like lithium (Li) and sodium (Na), known for their reactivity with water.
3B features transition metals, such as iron (Fe) and cobalt (Co). These elements often exhibit multiple oxidation states, contributing to their versatility in chemical reactions.
Lastly, 7A consists of halogens, including fluorine (F) and chlorine (Cl). Halogens are highly reactive nonmetals that form salts when combined with metals. Every element’s position reveals important information about its characteristics and potential interactions with others.
Understanding Groups and Periods
The periodic table organizes elements into groups and periods, which reveal key information about their properties. You can gain insights into chemical behavior by examining these classifications.
Definition of Groups
Groups are vertical columns on the periodic table. Each group contains elements that share similar chemical properties. For example:
- Group 1A: Includes alkali metals like lithium (Li) and sodium (Na). These elements react vigorously with water.
- Group 3B: Comprises transition metals such as iron (Fe) and cobalt (Co). They often show multiple oxidation states, enhancing their reactivity.
- Group 7A: Features halogens like fluorine (F) and chlorine (Cl). These nonmetals readily form salts when combined with metals.
Understanding groups helps predict how different elements will interact in reactions.
Definition of Periods
Periods are horizontal rows on the periodic table. Each period represents a new electron shell being filled as you move from left to right. For instance:
- Period 2 includes lithium (Li), beryllium (Be), boron (B), carbon (C), nitrogen (N), oxygen (O), fluorine (F), and neon (Ne).
- Period 3 consists of sodium (Na), magnesium (Mg), aluminum (Al), silicon (Si), phosphorus (P), sulfur (S), chlorine (Cl), and argon (Ar).
As you progress through a period, you’ll notice changes in properties such as electronegativity and atomic size. This structure aids in identifying trends across the periodic table.
Exploring 1A Elements
Group 1A elements, known as alkali metals, play a crucial role in chemistry due to their unique properties. These elements are highly reactive and typically found in nature only in compound form. Their reactivity increases down the group, making them fascinating for study.
Characteristics of Group 1A
Group 1A elements possess distinct characteristics that set them apart from other groups. They have one electron in their outermost shell, which they readily lose to form positive ions (cations). This configuration leads to high reactivity with water and oxygen. Additionally, these metals are soft and can be cut easily with a knife. They also exhibit low melting and boiling points compared to most metals.
Notable Examples of Group 1A Elements
Several notable elements belong to group 1A:
- Lithium (Li): The lightest metal known, lithium is widely used in batteries.
- Sodium (Na): Commonly found in table salt, sodium reacts vigorously with water.
- Potassium (K): Essential for biological functions, potassium helps regulate nerve impulses.
- Rubidium (Rb): Less common but still significant, rubidium has applications in atomic clocks.
- Cesium (Cs): Known for its use in timekeeping devices due to its precise atomic properties.
Each element showcases the typical behaviors of alkali metals while having unique applications that highlight their importance across various fields.
Exploring 3B Elements
Group 3B elements, also known as transition metals, play a significant role in various chemical reactions and applications. These elements often exhibit unique properties that make them versatile in different fields.
Characteristics of Group 3B
Group 3B elements possess distinctive characteristics that set them apart from other groups. They typically have multiple oxidation states, allowing them to participate in diverse chemical reactions. Additionally, they exhibit high melting and boiling points compared to lighter metals. Their ability to form colored compounds is noteworthy; for example, many transition metal ions produce vibrant colors when dissolved in solution. Also, their good conductivity of electricity makes these metals essential for electronic applications.
Notable Examples of Group 3B Elements
- Scandium (Sc): Used in aluminum alloys and sports equipment.
- Titanium (Ti): Known for its strength-to-weight ratio; widely used in aerospace.
- Vanadium (V): Essential for producing strong steel alloys.
- Chromium (Cr): Commonly found in stainless steel due to its corrosion resistance.
- Manganese (Mn): Vital for steel production and battery manufacturing.
These examples showcase how group 3B elements contribute significantly across industries while highlighting their unique properties and applications.
Exploring 7A Elements
Group 7A elements, known as halogens, exhibit unique characteristics and properties. These nonmetals are highly reactive due to their seven valence electrons. This reactivity makes them eager to gain one electron, forming stable compounds with metals. Halogens exist in various states at room temperature: fluorine (F) is a gas, bromine (Br) is a liquid, and iodine (I) is a solid.
Characteristics of Group 7A
Halogens possess distinct traits that set them apart from other groups. They have high electronegativity and ionization energies compared to other nonmetals. This means they attract electrons strongly during chemical reactions. Moreover, halogens typically form diatomic molecules (e.g., F2, Cl2), existing as pairs in gaseous forms. Their reactivity decreases down the group; for instance, fluorine reacts explosively with many substances while iodine’s reactions are milder.
Notable Examples of Group 7A Elements
Several notable examples illustrate the significance of group 7A elements:
- Fluorine (F): The most reactive element on the periodic table; it’s used in toothpaste and Teflon production.
- Chlorine (Cl): Commonly used as a disinfectant for water purification and in making plastics like PVC.
- Bromine (Br): A reddish-brown liquid utilized in flame retardants and certain medications.
- Iodine (I): Essential for thyroid function; found in iodized salt and antiseptics.
- Astatine (At): Rare and radioactive; it’s studied for potential medical applications despite its scarcity.
These examples highlight how each halogen plays an important role across various industries while also showcasing their individual properties.