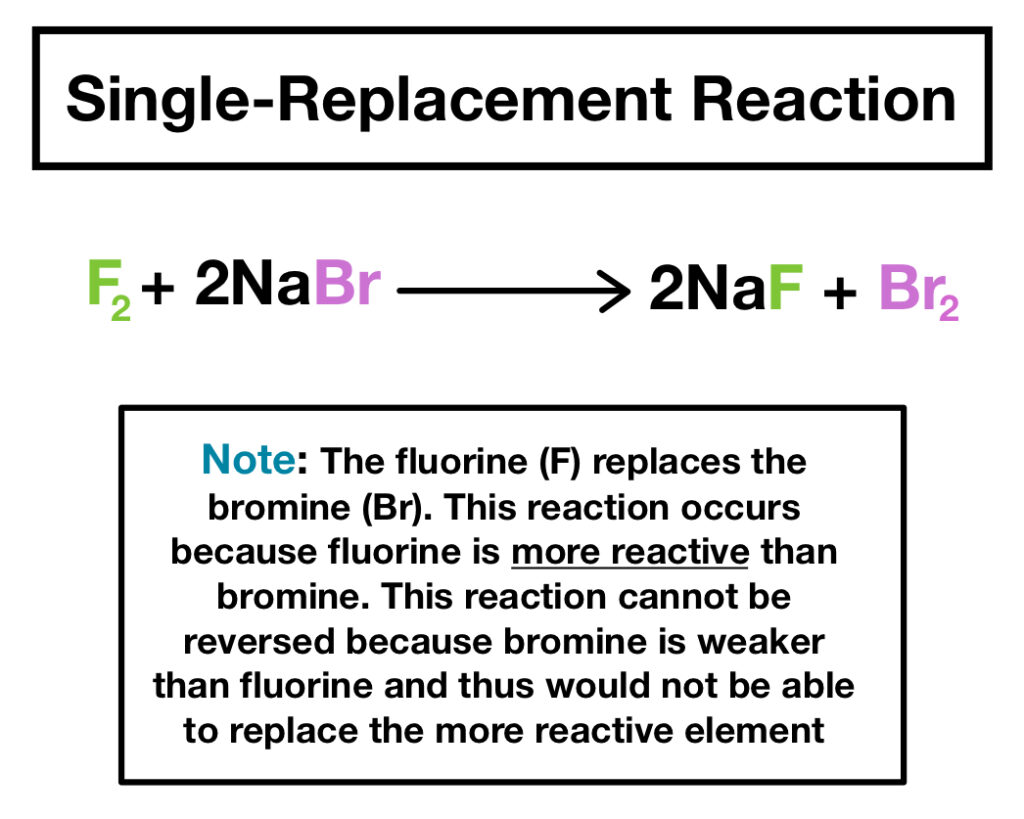
Have you ever wondered how certain elements can replace others in a chemical reaction? Single replacement reactions are fascinating processes that showcase the reactivity of metals and nonmetals. In these reactions, one element displaces another in a compound, leading to new substances and intriguing outcomes.
Overview Of Single Replacement Reactions
Single replacement reactions play a crucial role in chemistry. These reactions occur when one element replaces another in a compound, leading to the formation of new products. Understanding these reactions helps grasp fundamental chemical processes.
Definition And Importance
A single replacement reaction involves an element reacting with a compound, causing one component of the compound to be displaced. For instance, when zinc reacts with hydrochloric acid, it displaces hydrogen and forms zinc chloride and hydrogen gas. This type of reaction is significant as it demonstrates reactivity trends among elements and their ability to form new compounds.
General Formula
The general formula for a single replacement reaction can be expressed as:
[ A + BC rightarrow AC + B ]
In this equation, A represents the single element that displaces part of the compound BC, resulting in a new compound AC and releasing the displaced element B. For example:
- When iron (Fe) reacts with copper(II) sulfate (CuSO₄), you get iron(II) sulfate (FeSO₄) and copper (Cu).
This formula succinctly captures the essence of single replacement reactions while illustrating how elemental reactivity influences product formation.
Common Single Replacement Reaction Examples
Single replacement reactions frequently occur in both aqueous solutions and gas phases. Here are some noteworthy examples that illustrate these processes.
Examples In Aqueous Solutions
A common example occurs when zinc reacts with copper(II) sulfate in an aqueous solution. In this reaction, zinc displaces copper:
Zinc + Copper(II) Sulfate → Zinc Sulfate + Copper
This leads to the formation of zinc sulfate and solid copper. Another example involves iron reacting with hydrochloric acid:
Iron + Hydrochloric Acid → Iron(II) Chloride + Hydrogen Gas
In this case, hydrogen is displaced from the acid, creating iron(II) chloride and releasing hydrogen gas.
Examples In Gas Phase
In gas phase reactions, chlorine gas can react with sodium bromide. This results in a single replacement reaction where chlorine displaces bromine:
Chlorine + Sodium Bromide → Sodium Chloride + Bromine
Here, sodium chloride forms while bromine gas is released. Another notable reaction occurs when aluminum interacts with hydrochloric acid:
Aluminum + Hydrochloric Acid → Aluminum Chloride + Hydrogen Gas
The aluminum displaces hydrogen from the acid, generating aluminum chloride and releasing hydrogen gas into the atmosphere.
Factors Affecting Single Replacement Reactions
Single replacement reactions depend on various factors that influence their occurrence and efficiency. Understanding these elements enhances your grasp of chemical behavior in different scenarios.
Reactivity Of Elements
The reactivity of elements plays a crucial role in single replacement reactions. More reactive metals can displace less reactive ones from compounds. For example, when zinc (a more reactive metal) interacts with copper(II) sulfate, it successfully displaces copper, leading to the formation of zinc sulfate and solid copper. This trend follows the activity series of metals, where potassium ranks highest and gold ranks lowest in reactivity.
Environmental Conditions
Environmental conditions significantly affect the rate and outcome of single replacement reactions. Temperature influences reaction speed; higher temperatures typically increase kinetic energy, resulting in faster reactions. Additionally, concentration impacts how often reactants collide; for instance:
- Higher concentrations lead to more frequent collisions.
- Lower concentrations slow down reaction rates.
Pressure also matters for gas-phase reactions. Increasing pressure may shift equilibrium toward products if gaseous reactants are involved. Thus, you see how these conditions shape the dynamics of single replacement processes.
Applications Of Single Replacement Reactions
Single replacement reactions play a crucial role in various applications across different fields. Understanding these reactions leads to practical uses in industry and education.
Industrial Uses
Single replacement reactions are essential in metal extraction processes. For instance, zinc displaces copper from copper(II) sulfate to produce zinc sulfate and elemental copper. This method proves efficient for obtaining pure metals from their ores.
Another application involves the production of hydrogen gas. When aluminum reacts with hydrochloric acid, it generates aluminum chloride and releases hydrogen gas. Industries often harness this reaction for hydrogen production, which is vital for fuel cells and chemical synthesis.
In galvanization, iron structures undergo a single replacement reaction by coating them with zinc. This process protects iron from corrosion, extending the lifespan of metal products.
Educational Demonstrations
Single replacement reactions serve as excellent educational demonstrations. In a classroom setting, you can perform the classic experiment where magnesium ribbon reacts with hydrochloric acid to yield magnesium chloride and hydrogen gas. Observing bubbling provides a visual representation of gas evolution.
Moreover, mixing solutions of silver nitrate and sodium chloride demonstrates how silver displaces sodium in solution to form solid silver chloride precipitate. Such experiments engage students while illustrating fundamental chemical principles.
Additionally, demonstrating how zinc displaces copper showcases reactivity trends among metals effectively. It reinforces concepts such as the activity series and helps students grasp abstract ideas through hands-on experience.