Imagine a world where materials respond to magnetic fields in unexpected ways. Paramagnetic materials are those intriguing substances that exhibit such behavior, becoming magnetized only in the presence of an external magnetic field. This unique property sets them apart from their more familiar ferromagnetic counterparts.
In this article, you’ll discover fascinating examples of paramagnetic materials and their applications across various industries. From aluminum to certain metal oxides, these materials play a crucial role in technology and scientific research. Why should you care about paramagnetism? Understanding these materials can unlock new possibilities in electronics, medical imaging, and even environmental science.
Overview Of Paramagnetic Materials
Paramagnetic materials exhibit unique magnetic properties. They become magnetized only when exposed to an external magnetic field. This characteristic sets them apart from ferromagnetic materials that retain magnetization.
- Aluminum: This lightweight metal shows weak paramagnetism, making it useful in various applications.
- Manganese compounds: These often display significant paramagnetic behavior due to their unpaired electrons.
- Metal oxides: Certain oxides, like titanium oxide, possess paramagnetic properties and are critical in electronics.
- Transition metals: Elements such as iron and nickel can be found in paramagnetic forms, influencing many industrial processes.
These materials find practical uses in technology and research. For instance, they play a vital role in magnetic resonance imaging (MRI). Their responses to magnetic fields enhance imaging quality.
Understanding these materials enhances advancements across multiple fields. In electronics, for example, the utilization of aluminum improves device efficiency. In environmental science, manganese compounds aid in pollutant detection—demonstrating how crucial these substances are across diverse sectors.
Properties Of Paramagnetic Materials
Paramagnetic materials exhibit unique properties that differentiate them from other magnetic substances. They respond to external magnetic fields, becoming magnetized only in their presence. This behavior is crucial for various applications across technology and science.
Magnetic Susceptibility
Magnetic susceptibility characterizes the degree of magnetization in a material. Paramagnetic materials possess positive susceptibility values, meaning they align with an external magnetic field. Examples include:
- Aluminum: Exhibits weak paramagnetism, useful in lightweight applications.
- Manganese Compounds: Show higher susceptibility and are effective in sensors.
- Transition Metals: Certain forms of iron and nickel demonstrate paramagnetic characteristics.
High magnetic susceptibility indicates stronger interaction with magnetic fields, which can enhance device functionality.
Temperature Dependence
The temperature significantly impacts the paramagnetism of materials. As temperature rises, thermal motion disrupts alignment, reducing magnetization. For instance:
- Curie Temperature: Above this point, paramagnetic behavior transitions to non-magnetic.
- Example Materials:
- Manganese oxide maintains its properties up to certain temperatures before losing magnetization.
- Titanium oxide varies its response based on environmental conditions.
Understanding these factors aids in selecting materials for specific applications like medical imaging or electronic devices where precise magnetism is essential.
Applications Of Paramagnetic Materials
Paramagnetic materials play a crucial role in various fields, showcasing their unique properties. Their applications span across industries and research, making them invaluable.
In Industry
In the industrial sector, paramagnetic materials find extensive use due to their magnetic properties. For example:
- Aluminum enhances efficiency in electrical conductors and transformers.
- Manganese compounds serve as catalysts in chemical reactions.
- Titanium oxide is utilized in photocatalytic processes for environmental cleanup.
These materials contribute significantly to advancements in manufacturing and energy sectors. You might wonder how these applications impact everyday life; they improve product performance and reduce environmental footprints.
In Research
In scientific research, paramagnetic materials provide insights into fundamental physics and chemistry. They are often used in:
- MRI machines, where gadolinium-based contrast agents improve image quality.
- Electron spin resonance (ESR) experiments that study material properties at atomic levels.
- Magnetic susceptibility measurements, which help identify material characteristics.
Research utilizing these materials drives innovation across disciplines. Isn’t it fascinating how something so small can lead to discoveries that change our understanding of the world?
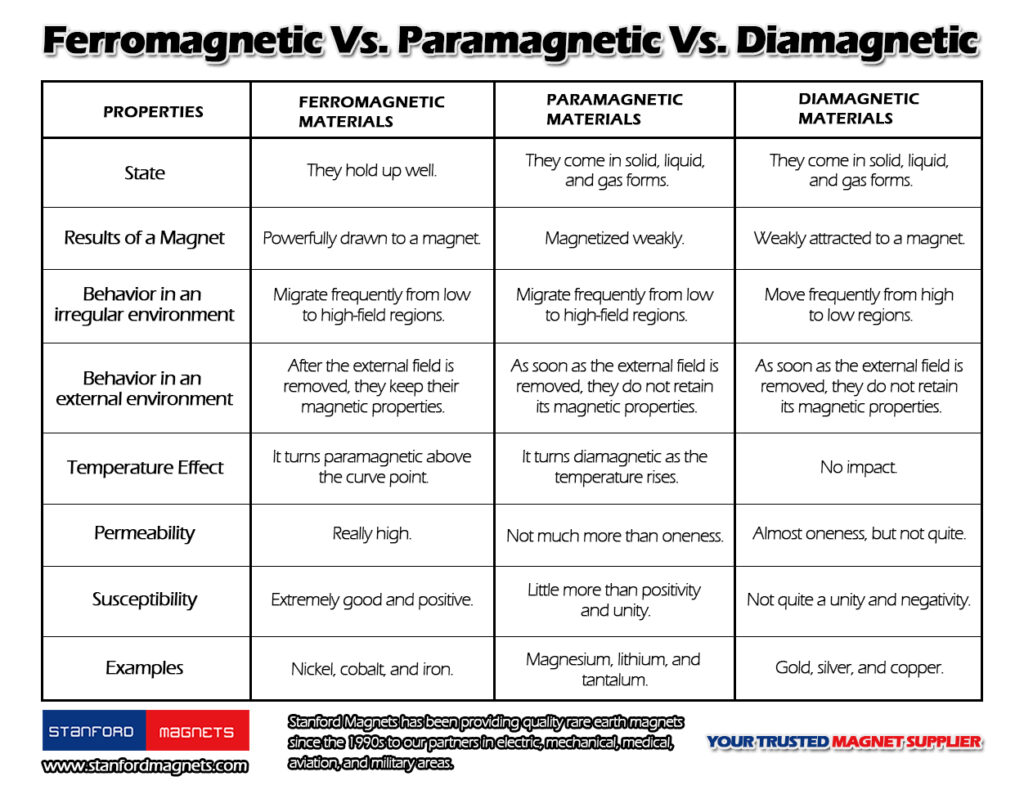
Comparison With Other Magnetic Materials
Paramagnetic materials differ significantly from other magnetic types. For instance, ferromagnetic materials like iron can retain magnetization even when the external field is removed. In contrast, paramagnetic substances lose their magnetization once the external magnetic field is gone.
Another category, diamagnetic materials, exhibits a weak repulsion in response to an external magnetic field. Unlike paramagnets, they possess negative susceptibility values and do not become magnetized under normal conditions.
Here are some examples highlighting these differences:
- Aluminum: As a paramagnetic material, aluminum shows weak magnetism only when exposed to a strong magnetic field.
- Iron and Nickel: These transition metals display ferromagnetism, retaining their magnetic properties without an external field.
- Copper: A classic example of diamagnetism, copper produces no net magnetization and instead opposes any applied magnetic field.
Understanding these distinctions enhances your grasp of how different materials respond to magnetic fields. Each type plays its own role across various applications in technology and research.